Understanding the Six-Pointed Shape of Snowflakes
Written on
Chapter 1: The Nature of Snowflakes
Snowflakes come in an astonishing variety of forms, with billions of unique designs. Despite their differences, they all share a common characteristic: a six-pointed structure. Let's explore the science behind this phenomenon.

What Are Snowflakes?
Contrary to popular belief, snowflakes are not simply frozen raindrops. They actually originate from sublimated water vapor. This means that moisture in the air transitions directly from gas to ice crystals without passing through a liquid state. This direct transition is referred to as sublimation.
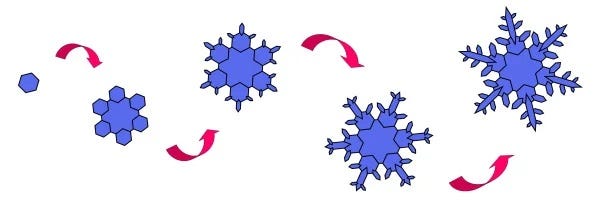
How Snowflakes Are Created
The process of snowflake formation starts with a tiny hexagonal plate. As the snowflake descends through the atmosphere, sublimation continues, causing new ice crystals to accumulate at the corners of the initial hexagon. This results in each snowflake being one-of-a-kind; even minor variations in temperature, humidity, and wind can significantly influence the growth of the ice crystals.
Although the six branches of a single snowflake usually develop in a similar manner, complete symmetry is quite rare. Slight variations can exist among the branches, but it is possible to create identical snowflakes in a controlled laboratory environment.
Why Six Points?
The six-pointed shape of snowflakes is rooted in the geometry of the ice crystal lattice.
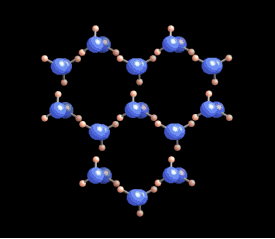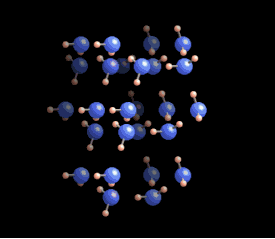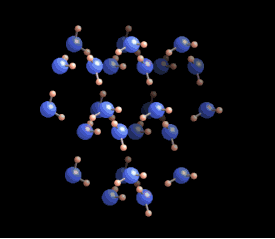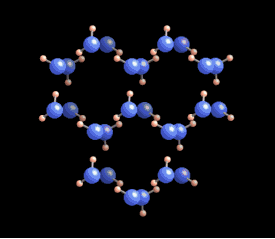
A water molecule is composed of two hydrogen atoms and one oxygen atom. The arrangement of these atoms leads to a positive charge on the hydrogen and a negative charge on the oxygen. This polarity allows water molecules to form hydrogen bonds with each other.
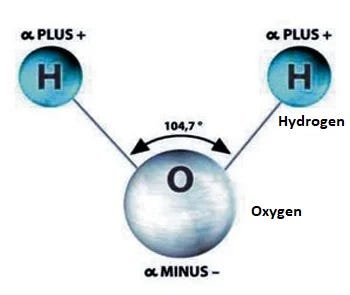
In liquid form, hydrogen bonds are constantly being created and broken due to higher temperatures. However, as water cools to around zero degrees Celsius, the energy levels decrease, allowing for more stable hydrogen bonds to develop. Each water molecule can connect with up to four other molecules through these bonds.
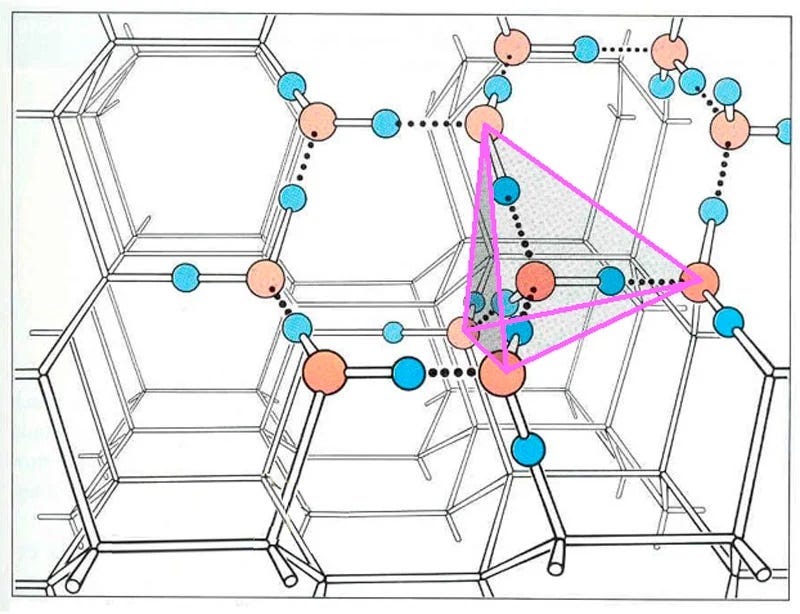
The molecules within an ice crystal tend to adopt the most energetically favorable configuration under pressure, which is tetrahedral in shape. In this arrangement, each vertex of the tetrahedron is occupied by a water molecule that is bonded to others. Various stable structures can emerge from tetrahedrons, but the hexagonal structure is the most stable and energy-efficient. This type of ice is known as Ih (where "h" stands for hexagonal) ice, which constitutes about 99% of Earth's ice.

While alternative forms of ice exist, they typically require distinct formation conditions. For instance, cubic ice (Ic) can form in the upper atmosphere where temperatures and pressures are significantly lower.
In summary, the hexagonal shape of snowflakes is fundamentally linked to the hexagonal arrangement of the ice crystal lattice.
Subscribe to our channel for more engaging content about the wonders of nature! If you're interested in supporting our work, consider becoming a member for just $5 a month to help us improve our content.