The Ethical Dilemmas of CRISPR Gene Editing: A Closer Look
Written on
Chapter 1: Introduction to CRISPR Technology
One year ago, the scientific community was shaken by the birth of the world’s first genetically modified babies—twin girls known as Lulu and Nana. Chinese researcher He Jiankui utilized CRISPR technology to alter the CCR5 gene in embryos, aiming to provide resistance against HIV. This unprecedented event led to significant outrage globally, prompting China to revise its civil code to enforce accountability for adverse effects resulting from genetic modifications in humans. Recently, reproductive scientists at Weill Cornell Medicine in New York City have embarked on their own controversial project targeting the BRCA2 gene in sperm. However, before we hastily venture into reshaping our genetic futures, it’s crucial to reassess the risks and implications associated with gene editing.
As we delve into the capabilities of CRISPR-Cas9, it’s vital to grasp how this technology operates.
Section 1.1: Understanding CRISPR-Cas9 Mechanisms
CRISPR-Cas9 leverages a bacterial defense mechanism akin to adaptive immunity. CRISPR, an acronym for clustered regularly interspaced short palindromic repeats, serves as an RNA-based defense against viral DNA in bacteria. When a bacterium encounters a virus, it retains snippets of the viral DNA in its genome, enabling it to recognize and combat future infections. Essentially, these genetic snippets act as memory markers that empower bacteria to identify and slice through matching viral DNA when necessary.
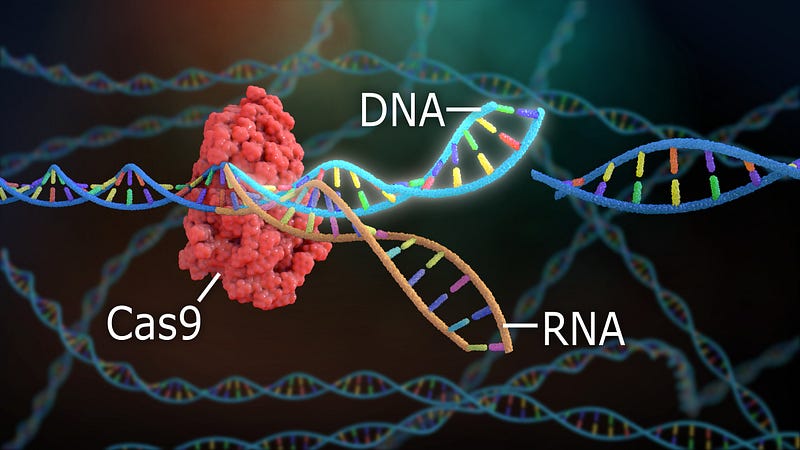
In 2012, Jennifer Doudna and Emmanuelle Charpentier showcased how CRISPR could be employed to target specific DNA sequences. The CRISPR-Cas9 system not only allows for the identification and removal of DNA sequences but also enables modifications. Following the completion of the Human Genome Project in 2003, which mapped our genetic framework, CRISPR emerged as a tool to amend or delete specific genetic segments. This technology holds the potential to treat blood disorders like sickle cell anemia, which stems from a single genetic mutation.
Nevertheless, this newfound capability raises significant ethical questions. A primary concern among scientists is the long-term effects of germline modifications—genetic alterations made in human eggs, sperm, or embryos. Such changes impact every cell within an organism and are inheritable, meaning any unintended consequences could persist for generations. Germline modifications are essentially permanent.
The possibility of human germline alteration also paves the way for designer babies, enabling not only the correction of genetic disorders but also the introduction of traits that could protect against diseases like Alzheimer’s or even slow down aging. This potential for controlling our genetic destinies raises alarms reminiscent of dystopian narratives, like those found in "Frankenstein" or "Brave New World." Consequently, in 2015, Doudna and other scientists advocated for a moratorium on human genome editing using CRISPR-Cas9 until safety and efficacy concerns could be adequately addressed.
Chapter 2: The Reality of CRISPR’s Impact
The first video, "The Realities of Gene Editing with CRISPR I NOVA I PBS," explores the implications and ethical considerations surrounding the use of CRISPR technology.
CRISPR is currently being explored in clinical trials for cancer and blood disorders. Unlike Dr. He’s experiment, these trials don’t produce heritable DNA changes, yet they still carry inherent risks. Skepticism remains regarding the safety and effectiveness of CRISPR, echoing the fate of many previously promising technologies. Historical gene therapies, which aimed to introduce healthy gene copies into cells via viruses, faced early challenges, including the tragic death of Jesse Gelsinger during a trial in 1999.
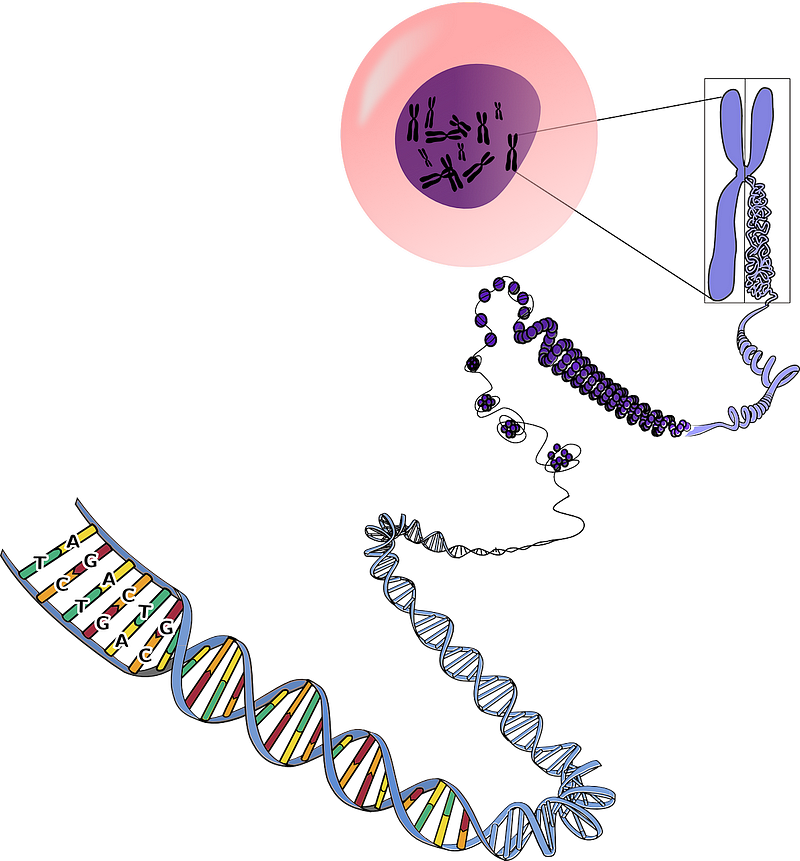
Although CRISPR is generally more precise than traditional gene therapies, it can still result in unintended genetic alterations, particularly in closely linked gene pairs. Errant edits to one gene in a linked set might inadvertently impact its neighbor. Moreover, even deliberate modifications can yield unforeseen outcomes. Research published in 2018 in Nature Medicine indicated that CRISPR edits could heighten cancer risks by inhibiting the tumor suppressor gene P53, dubbed “the guardian of the genome” for its role in preserving genomic integrity. This gene’s repair mechanisms can either fix cellular damage or trigger cell death. Thus, successful edits could necessitate P53 inhibition, potentially increasing vulnerability to mutations that lead to cancer.
“We don’t always fully understand the changes we’re making,” states Alan Regenberg, a bioethicist at Johns Hopkins Berman Institute of Bioethics. “Even if we achieve our desired edits, there’s still uncertainty about their effects.”
Additionally, recent research may not undergo the usual rigorous scrutiny by the National Institutes of Health, raising further safety concerns.
Even if CRISPR technology becomes sufficiently accurate, the notion of classifying genes as either beneficial or harmful is fundamentally flawed. Genetic strength or weakness is contextual; traits perceived as disadvantages may provide resilience in changing environments.
“It is an error to imagine that evolution signifies a constant tendency to increased perfection. That process undoubtedly involves a constant remodeling of the organism in adaptation to new conditions; but it depends on the nature of those conditions whether the direction of the modifications effected shall be upward or downward. Retrogressive is as practicable as progressive metamorphosis.”
— English Biologist Thomas Henry Huxley, 1888

In biological terms, organisms most adapted to their environments exhibit the highest fitness, which includes both survival and reproductive success. While accumulating mutations over time can lead to various diseases, genetic diversity can also provide advantages in unfamiliar or stressful situations, such as drought or disease outbreaks. Discussions about rigid natural selection should evolve into more nuanced conversations about “balancing selection,” which favors genetic diversity over the fixation of a single ideal variant, as noted by Professor Maynard V. Olson at the University of Washington.
Evolution has allowed many potentially harmful genes to persist in the gene pool because they can confer selective advantages to carriers, a phenomenon known as heterozygote advantage. For instance, sickle cell anemia, which is inherited in an autosomal recessive manner, requires two copies of the variant for disease expression. However, possessing just one copy provides resistance to malaria, explaining the variant’s higher prevalence in malaria-endemic regions like India and parts of Africa.
To illustrate the benefits of genetic diversity, consider that over half of all human liver cells are polyploid, possessing multiple copies of chromosomes. While such abnormalities are often viewed as defects, they may actually confer resilience against DNA-damaging agents. If one chromosome in a liver cell sustains damage, backup copies can ensure proper gene function.
Harvard geneticist George Church presents anecdotal evidence supporting the value of neurodiversity, sharing insights on how individuals with autism, often labeled as intellectually disabled, may contribute unique perspectives and innovations. Church himself has navigated challenges such as narcolepsy and dyslexia, which he believes have enhanced his creativity.

The Yale Center for Dyslexia & Creativity reports that dyslexia affects about 20% of the U.S. population and comprises 80-90% of individuals with learning disabilities. A study by Cass Business School found a higher prevalence of dyslexia among entrepreneurs, with 35% of U.S. entrepreneurs displaying dyslexic traits. This study suggests that compensatory skills, such as effective delegation and enhanced communication, may contribute to entrepreneurial success.
Investors from Shark Tank, including Barbara Corcoran and Kevin O’Leary, have spoken about how dyslexia has shaped their competitive edge and creativity. Richard Branson and Ikea founder Ingvar Kamprad also credit their dyslexic experiences with fostering innovation and unique perspectives. Thus, misguided attempts to normalize the genetic spectrum could overlook the valuable contributions of genetic neurodiversity.
Chapter 3: The Future of Genetic Editing
The second video, "Genome Editing with CRISPR-Cas9," further delves into the scientific advancements and societal implications of gene editing technologies.
The Case Against CRISPR Gene Editing, Part II
On designer babies, gene reference libraries, and more
Follow Medical Myths and Models on Medium and sign up for our mailing list to stay connected!